Abstract
Background Anti-PD1 inhibition is increasingly investigated as first-line treatment of classical Hodgkin lymphoma (HL), usually either in sequential or concomitant combination with other therapeutics. While impressive response rates and short-term progression-free survival are observed, long-term efficacy and safety as well as quality-of-life (QoL) remain unknown. Herein we present the preplanned 3-year follow-up (FU) analysis of the GHSG phase II NIVAHL trial in early-stage unfavorable HL.
Methods NIVAHL is an investigator-sponsored randomized phase II trial (NCT03004833) financially supported by Bristol-Myers Squibb which was conducted at 28 GHSG trial centers and enrolled 109 patients aged 18-60 years with centrally confirmed first diagnosis of early-stage unfavorable HL. Patients received either fully concomitant (4xnivo-AVD; arm A) or sequential (4xnivolumab, 2xnivo-AVD, 2xAVD; arm B) nivolumab-based 1st-line treatment, each followed by 30Gy involved-site radiotherapy (IS-RT). The primary analysis showed feasibility with a favorable safety profile and outstanding 1-year efficacy (Bröckelmann JAMA Oncol 2020). During FU, patients were monitored regularly for signs of HL relapse, persisting or late toxicities and QoL by the EORTC QLQ-C30 questionnaire. This FU analysis of key secondary endpoints including progression-free (PFS) and overall survival (OS), long-term safety and QoL was preplanned at three years after the registration of the last patient.
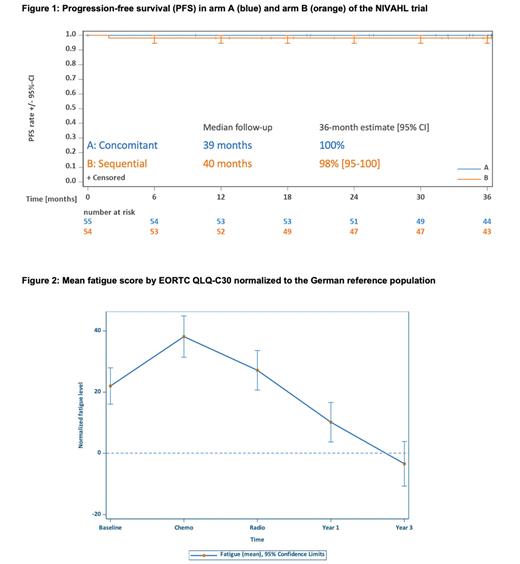
Results With a median FU of 40 months, no PFS or OS events were observed since the NIVAHL primary analysis. Importantly, no patient with a partial remission by central response assessment required further treatment and all 7 patients converted to a complete remission during FU. With no deaths recorded, 3-year OS is 100% in both arms and 3-year PFS estimates are 98% and 100% in arm A and B, respectively (Figure 1). No second primary malignancies were reported and overall, no new safety signals were identified: While any treatment for potentially treatment-associated morbidities was required in 13% of patients, no patient required long-term corticosteroid treatment at last FU. Mean forced expiratory pressure in one second (FEV1) at baseline and 3 years after enrollment was 91.1% (standard deviation (SD) 13.6%) and 94.7% (SD 13%), respectively. Mean diffusion capacity for carbon monoxide adjusted for hemoglobin (DLCOc) at baseline and 3 years after enrollment was 86.2% (SD 16.5%) and 81.0% (SD 22.6%), respectively. No cardiac events >°1 were reported during FU and left ventricular ejection fraction (LVEF) was in the normal range in 93% of patients at last FU. The median global health score and global QoL by EORTC QLQ-C30 improved over time and were 64 (SD 22) and 80 (SD 16) at baseline, 76 (SD 17) and 87 (SD 15) 1 year after enrollment and 82 (SD 15) and 92 (SD 11) 3 years after enrollment, respectively. Relevant fatigue existed prior to treatment (mean fatigue score difference from age- and sex-adjusted German population reference value: 22 (SD 30)), briefly worsened on-treatment (after chemotherapy: 38 (SD 27), after radiotherapy: 27 (SD 23)) but resolved over time (1 year after enrollment: 10 (SD23), 3 years after enrollment: -3 (SD 18); Figure 2).
Conclusion This preplanned FU analysis of the largest anti-PD1 HL first-line trial to date confirms the outstanding efficacy and favorable safety profile of this therapeutic approach. Additionally, QoL and fatigue appear relevantly improved compared to baseline during prolonged FU. Building on NIVAHL, the upcoming GHSG phase II INDIE trial (NCT04837859) will investigate an individualized immunotherapy with tislelizumab in early-stage unfavorable HL patients. Since likely not all patients require the full course of chemoimmunotherapy and consolidative IS-RT, INDIE will be the first trial investigating a chemo- and radiotherapy-free 1st-line HL treatment in optimally responding patients.
Disclosures
Bröckelmann:Celgene: Other: Travel Support; Takeda: Consultancy, Honoraria, Research Funding; MSD: Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding. Kerkhoff:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees; Eusa Pharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sobi: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Borchmann:Miltenyi Biotec: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novarts: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. von Tresckow:Abbvie: Other: NA; Allogene: Consultancy; Amgen: Consultancy; AstraZeneca: Honoraria, Other: NA; BMS: Honoraria, Other: NA; Cerus: Consultancy; Gilead/Kite: Consultancy, Honoraria, Other: NA, Research Funding; Incyte: Consultancy, Honoraria; IQVIA: Consultancy; Miltenyi: Consultancy; MSD: Consultancy, Honoraria, Other: NA, Research Funding; Novartis: Consultancy, Honoraria, Other: NA, Research Funding; Pentixafarm: Consultancy; Pfizer: Consultancy; Roche: Consultancy, Honoraria, Other: NA; Takeda: Consultancy, Honoraria, Other: NA, Research Funding.
OffLabel Disclosure:
Nivolumab in sequential or concomitant combination with AVD as 1st-line treatment of classical Hodgkin lymphoma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal